Short Communication |
|
Corresponding author: Michele Luca Geraci ( micheleluca.geraci@gmail.com ) Academic editor: Paraskevi Karachle
© 2022 Giacomo Sardo, Michele Luca Geraci, Fabio Falsone, Salvatore Gancitano, Vita Gancitano, Danilo Scannella, Charles Odilichukwu R. Okpala, Antonino Titone, Sergio Vitale.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Sardo G, Geraci ML, Falsone F, Gancitano S, Gancitano V, Scannella D, Okpala COR, Titone A, Vitale S (2022) First record and otolith morphometric description of an adult lightfish, Ichthyococcus ovatus (Actinopterygii: Stomiiformes: Phosichthyidae), caught in the Strait of Sicily (central Mediterranean Sea). Acta Ichthyologica et Piscatoria 52(2): 159-166. https://doi.org/10.3897/aiep.52.84928
|
Abstract
On July 2018, one specimen of Ichthyococcus ovatus (Cocco, 1838) was caught in the Strait of Sicily during the International Bottom Trawl Survey in the Mediterranean (MEDITS). The adult I. ovatus measured 49 mm in total length and weighed 1.44 g. In this context, the presently reported study constitutes the first and deepest record of an adult of I. ovatus as well as the morphometric description of its sagittal otoliths. In addition, we provide an age estimation as well as an update of the geographical distribution of this bathypelagic species around the Mediterranean Sea. Based on the growth increments of sagittal otoliths, the estimated age was five years. Specifically, the otolith from the presently reported specimen of I. ovatus tended to be elliptic in shape related to aspect ratio and high rectangularity while circularity showed high complexity of otolith contour complexity. The absence of economic value of rarely reported species may underestimate their abundance. Therefore, more studies and research surveys would be necessary to fill the information gap on the biology of these deep-water species.
Keywords
Mediterranean deep sea, otolith, rare species, MEDITS, Strait of Sicily, trawl survey
Introduction
The family Phosichthyidae of the order Stomiiformes (
In relation to the Mediterranean Sea, the authors herein could only find the study of
Apart from the geographical distribution and nictemeral migration, the biological information about lightfishes appears limited. Furthermore, relevant information regarding the otoliths of I. ovatus specific to the Strait of Sicily (central Mediterranean Sea), to our best knowledge, is not available. Therefore, to supplement existing information, the presently reported study presents the first record and otolith morphometric description of an adult lightfish, I. ovatus, caught in the Strait of Sicily. In addition, we provide an age estimation as well as an update of the geographical distribution of this bathypelagic species around the Mediterranean Sea.
Materials and methods
Sample collection, identification, and biometrics. On July 2018, one specimen of Ichthyococcus ovatus (trawl haul points: 36°36.89′N, 013°21.24′E) was caught, at a depth of about 547 m, during the International Bottom Trawl Survey in the Mediterranean (MEDITS) (
- entire ventral photophores row extending from anterior end of isthmus to posterior termination of this row on caudal peduncle (IC);
- ventral series of pelvic and anal photophores, part of IC extending between a vertical line at insertion of posterior pelvic fin ray and anal fin origin or to end of row (VAV + AC);
- entire lateral series photophores on body side (OA).
Age estimation and otolith morphometry. The otoliths’ extraction was performed based on the procedures recommended by
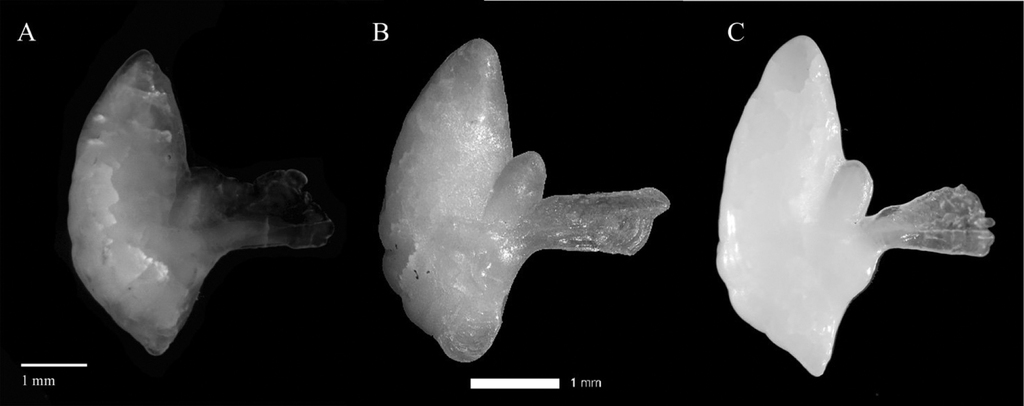
(A) Distal surface of the sagittal otoliths from Ichthyococcus ovatus. (B) Enhanced image of the right otolith used to count presumptive annuli for age estimation. Black dots represent the growth rings; the distance between a and b is the otolith width while the distance between c and d is the otolith length; (C), proximal surface of the left otolith showing rostrum (R), antirostrum (A), excisura ostii (E), sulcus acusticus (SA, continuous line), colliculum ostii (CO, dotted line), colliculum caudii (CC, dashed line).
The morphometric data of the otoliths were collected, which included area (Ao), perimeter (Po), length (Lo, maximal distance from the anterior tip to the posterior edge, parallel to the sulcus (
Geographical distribution and mapping. The geographical distribution of this lightfish species has been prepared by compiling all existing scientific literature concerning reported records of I. ovatus with particular reference to the Mediterranean Sea. Every published article we found that contained reports of I. ovatus in the Mediterranean Sea was scrutinized in order to extract the spatial data. In addition, the Mediterranean records of this species lacking in the literature were found using the Global Biodiversity Information Facility (
Results
The photographic image of the Ichthyococcus ovatus specimen caught in the Strait of Sicily is shown in Fig.
Comparison of biometric and meristic characters of the presently reported Ichthyococcus ovatus from the Strait of Sicily with those provided by selected literature sources.
| Character | This paper |
|
|
||||
|---|---|---|---|---|---|---|---|
| n = 1 | n = 1 | n = 40 | |||||
| Absolute | Relative | Meristic | Absolute | Absolute | |||
| [mm] | [g] | [%SL] | mm | mm | [g] | ||
| Total length | 59 | 45 | |||||
| Standard length | 49 | 16.9–38.1 | |||||
| Head length | 14 | 28.6 | |||||
| Eye diameter | 4 | 8.2 | |||||
| Total weight | 1.44 | 0.11–1.27 | |||||
| Dorsal fin length | 9 | 18.4 | |||||
| Pectoral fin length | 7 | 14.3 | |||||
| Ventral fin length | 4 | 8.2 | |||||
| Anal fin length | 7 | 14.3 | |||||
| Dorsal fin rays | 11 | ||||||
| Pectoral fin rays | 8 | ||||||
| Ventral fin rays | 7 | ||||||
| Anal fin rays | 16 | ||||||
| Vertebrae | 42 | ||||||
| IC | 46 | ||||||
| VAV + AC | 21 | ||||||
| OA | 23 | ||||||
The examination of the whole otoliths by the distal surface as shown in Fig.
Shape parameters and indices from otolith of Ichthyococcus ovatus from the Strait of Sicily, described in the presently reported study.
| Shape parameters | Value |
|---|---|
| Area (Ao) [mm2] | 8.89 |
| Perimeter (Po) [mm] | 17.01 |
| Mass (Mo) [mg] | 0.0154 |
| Length (Lo) [mm] | 3.99 |
| Width (Wo) [mm] | 4.68 |
| Shape indices | |
| Otolith relative length (TL) | 6.76 |
| Otolith relative length (SL) | 8.14 |
| Otolith relative size | 2.55 |
| Aspect ratio (Ar) | 0.85 |
| Form factor (Ff) | 0.39 |
| Ellipticity (El) | 0.07 |
| Roundness (Ro) | 0.71 |
| Rectangularity (Re) | 0.48 |
| Circularity (Ci) | 32.54 |
Overall, the otolith of the presently reported study tended to be elliptic in shape related to aspect ratio (Ar) and high rectangularity (Re) while circularity (Ci) showed high complexity of otolith contour (Table
Map comparing the geographical distribution of I. ovatus of the presently reported study with those of other previous studies within the Mediterranean Basin is shown in Fig.
Map showing the geographical distribution of Ichthyococcus ovatus based on the previous and the presently reported study within the Mediterranean Basin. Specific records include: green, blue, violet, pink, stripes, and green pentagon as larvae; brown and light green stripes as the probable catch areas for specimens of Libyan, and Egyptian waters, respectively.
Discussion
Consistent with the features described by
Environmental factors are believed to influence the otolith shape such as the depth, temperature, substrate type, salinity, and feeding conditions (
The widespread nature of this lightfish species is demonstrated by its geographical distribution within the Mediterranean Sea. The records in the waters off Libya (
Conclusions
The first record and morphometric description of sagittae otoliths in an adult Ichthyococcus ovatus specific to the Strait of Sicily has been presented in this communication. It also included an updated geographical distribution of this deep-water species around the Mediterranean Sea. As we have considered the putative age of the I. ovatus specimen estimated at five years, the periodicity in the formation of the rings must be established and age validation studies are required for accurate age determination of this lightfish species. This presently reported study is preliminary and lays a baseline for the future study of this I. ovatus species, which are not commonly caught by trawling likely because of its bathymetric distribution. A more robust study involving age validation and shape analysis will require the collection of more I. ovatus species samples. Indeed, the absence of economic value of rarely reported species may actually underestimate their presence/abundance in the Mediterranean basin (
Acknowledgments
This work is conducted thanks to the European Data Collection Framework (DCF)—MEDITS survey module—funded by the European Union and the Italian Ministry for Agricultural, Food, and Forestry Policies.
References
- Ahlstrom EH, Ball OP (1954) Description of eggs and larvae of jack mackerel (Trachurus symmetricus) and distribution and abundance of larvae in 1950 and 1951. Fishery Bulletin 56: 209–245.
- Ahlstrom EH, Richards WJ, Weitzman SH (1984) Families Gonostomatidae, Sternoptychidae, and associated stomiiform groups: Development and relationships. Pp. 184–198. In: Moser HG, Richards WJ, Cohen DM, Fahay MP, Kendall AWJr, Richardson SL (eds) Ontogeny and systematics of fishes based on and international symposium dedicated to the memory of Elbert Halvor Ahlstrom. The symposium was held August 15–18, 1983, La Jolla, California. American Society of Ichthyologists and Herpetologists. Special Publication No. 1.
- Akel EH, Karachle PK (2017) The marine ichthyofauna of Egypt. Egyptian Journal of Aquatic Biology and Fisheries 21(3): 81–116. https://doi.org/10.21608/ejabf.2017.4130
- Badcock J (1984) Photichthyidae. P. 510. In: Whitehead PJP, Bauchot ML, Hureau JC, Nielsen J, Tortonese E (Eds) Fishes of the North-eastern Atlantic and the Mediterranean. UNESCO 1.
- Battaglia P, Malara D, Romeo T, Andaloro F (2010) Relationships between otolith size and fish size in some mesopelagic and bathypelagic species from the Mediterranean Sea (Strait of Messina, Italy). Scientia Marina 74(3): 605–612. https://doi.org/10.3989/scimar.2010.74n3605
- Bertrand JA, Gil de Sola L, Papaconstantinou C, Relini G, Souplet A (2002) The general specifications of the MEDITS surveys. Scientia Marina 66(S2): 9–17. https://doi.org/10.3989/scimar.2002.66s29
- Biagi F, Sartor P, Ardizzone GD, Belcari P, Belluscio A, Serena F (2002) Analysis of demersal fish assemblages of the Tuscany and Latium coasts (north-western Mediterranean). Scientia Marina 66(S2): 233–242. https://doi.org/10.3989/scimar.2002.66s2233
- Cadrin SX, Friedland KD (2005) Morphometric outlines. Pp. 173–184. In: Cadrin SX, Friedland KD, Waldman JR (Eds) Stock identification methods: applications in fishery science. Elsevier, Amsterdam. https://doi.org/10.1016/B978-012154351-8/50009-5
- Campana SE, Thorrold SR (2001) Otoliths, increments, and elements: Keys to a comprehensive understanding of fish populations? Canadian Journal of Fisheries and Aquatic Sciences 58(1): 30–38. https://doi.org/10.1139/f00-177
- Campana SE (2005) Otolith elemental composition as a natural marker of fish stocks. Pp. 227–245. In: Cadrin SX, Friedland K, Waldman JR (Eds) Stock identification methods: applications in fishery sciences. Elsevier, Amsterdam, the Netherlands. https://doi.org/10.1016/B978-012154351-8/50013-7
- Chilton DE, Beamish RJ (1992) Age determination methods for fishes studied by the groundfish program at the Pacific Biological Station. Canadian Special Publication of Fisheries and Aquatic Sciences, Ottawa, 60: 102 pp.
- Clarke GL (1971) Light conditions in the sea in relation to the diurnal vertical migrations of animal). Pp. 41–50. In: Farquaar GB (Ed) (1971). Proceedings of the international symposium on biological sound scattering in the ocean. Maury Center Ocean Science, Washington, DC, USA.
- Cuttitta A, Arigo A, Basilone G, Bonanno A, Buscaino G, Rollandi L, Garcia Lafuente J, Garcia A, Mazzola S, Patti B (2004) Mesopelagic fish larvae species in the Strait of Sicily and their relationships to main oceanographic events. Hydrobiologia 527(1): 177–182. https://doi.org/10.1023/B:HYDR.0000043299.65829.2f
- Elbaraasi H, Elabar B, Elaabidi S, Bashir A, Elsilini O, Shakman E, Azzurro E (2019) Updated checklist of bony fishes along the Libyan coasts (southern Mediterranean Sea). Mediterranean Marine Science 20(1): 90–105. https://doi.org/10.12681/mms.15570
- Falsone F, Geraci ML, Scannella D, Okpala COR, Giusto GB, Bosch-Belmar M, Bono G (2017) Occurrence of two rare species from order Lampriformes: Crestfish Lophotus lacepede (Giorna, 1809) and scalloped ribbonfish Zu cristatus (Bonelli, 1819) in the northern coast of Sicily, Italy. Acta Adriatica 58(1): 137–146. https://doi.org/10.32582/aa.58.1.11
- Froese R, Pauly D (eds.) 2022. FishBase. [Version 02/2022] http://www.fishbase.org
- GBIF (2022) Global Biodiversity Information Facility.
- Geraci ML, Di Lorenzo M, Falsone F, Scannella D, Di Maio F, Colloca F, Vitale S, Serena F (2019) The occurrence of Norwegian skate, Dipturus nidarosiensis (Elasmobranchii: Rajiformes: Rajidae), in the Strait of Sicily, central Mediterranean. Acta Ichthyologica et Piscatoria 49(2): 203–208. https://doi.org/10.3750/AIEP/02566
- Goçalo CG, Katsuragawa M, Silveira ICAD (2011) Patterns of distribution and abundance of larval Phosichthyidae (Actinopterygii, Stomiiformes) in southeastern Brazilian waters. Brazilian Journal of Oceanography 59(3): 213–229. https://doi.org/10.1590/S1679-87592011000300002
- Granata A, Cubeta A, Minutoli R, Bergamasco A, Guglielmo L (2011) Distribution and abundance of fish larvae in the northern Ionian Sea (Eastern Mediterranean). Helgoland Marine Research 65(3): 381–398. https://doi.org/10.1007/s10152-010-0231-2
- Harvey JT, Oughlin TR, Perez MA, Oxman DS (2000) Relationship between fish size and otolith length for 63 species of fishes from the eastern North Pacific Ocean. NOAA Technical Report 150: 1–36.
- Lin CH, Chiang YP, Tuset VM, Lombarte A, Girone A (2018) Late Quaternary to Recent diversity of fish otoliths from the Red Sea, central Mediterranean, and NE Atlantic sea bottoms. Geobios 51(4): 335–358. https://doi.org/10.1016/j.geobios.2018.06.002
- Lombarte A, Lleonart J (1993) Otolith size changes related with body growth, habitat depth and temperature. Environmental Biology of Fishes 37(3): 297–306. https://doi.org/10.1007/BF00004637
- Lombarte A, Chic O, Parisi-Baradad V, Olivella R, Piera J, Garca-Ladona E (2006) A web-based environment from shape analysis of fish otoliths. The AFORO database. Scientia Marina 70(1): 147–152. https://doi.org/10.3989/scimar.2006.70n1147
- Morat F, Letourneur Y, Nérini D, Banaru D, Batjakas IE (2012) Discrimination of red mullet populations (Teleostean, Mullidae) along multi-spatial and ontogenetic scales within the Mediterranean basin on the basis of otolith shape analysis. Aquatic Living Resources 25(1): 27–39. https://doi.org/10.1051/alr/2011151
- Mytilineou C, Politou CY, Papaconstantinou C, Kavadas S, D’Onghia G, Sion L (2005) Deep-water fish fauna in the Eastern Ionian Sea. Belgian Journal of Zoology 135(2): 229–233.
- Olivar MP, Bernal A, Molí B, Peña M, Balbín R, Castellón A, Miquel J, Massutí E (2012) Vertical distribution, diversity and assemblages of mesopelagic fishes in the western Mediterranean. Deep-sea Research. Part I, Oceanographic Research Papers 62: 53–69. https://doi.org/10.1016/j.dsr.2011.12.014
- Ozpicak M, Saygin S, Aykut Yydin A, Hancer E, Savaşyilmaz S, Polat N (2018) Otolith shape analyses of Squalius cephalus (Linnaeus, 1758) (Actinopterygii: Cyprinidae) inhabiting four inland water bodies of the middle Black Sea region, Turkey. Iranian Journal of Ichthyology 5(4): 293–302.
- Palomera I, Rubiés P (1979) Ichthyoplancton de la mer Catalane. Larves de poissons récoltées sur deux stations fixes devant Barcelona au cours d’un cycle annuel (1975–1976). Commission Internationale pour l’Exploration Scientifique de la mer Méditerranée: Comité des vertébrés marins et céphalopodes (1978). Rapports et procès-verbaux des réunions 26/27(10): 201–206.
- Papaconstantinou C (1990) Some rare mesopelagic and bathyal fish caught in the Greek seas. HCMR, Athens.
- Pavlov DA (2016) Differentiation of three species of the genus Upeneus (Mullidae) based on otolith shape analysis. Journal of Ichthyology 56(1): 37–51. https://doi.org/10.1134/S0032945216010094
- Popper AN, Ramcharitar J, Campana SE (2005) Why otoliths? Insights from inner ear physiology and fisheries biology. Marine and Freshwater Research 56(5): 497–504. https://doi.org/10.1071/MF04267
- QGIS Development Team. 2020. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org
- Rodríguez JM, Alvarez I, López-Jurado JL, Garcia A, Balbín R, Álvarez-Berastegui D, Torres AP, Alemany F (2013) Environmental forcing and the larval fish community associated to the Atlantic bluefin tuna spawning habitat of the Balearic region (Western Mediterranean), in early summer 2005. Deep-sea Research. Part I, Oceanographic Research Papers 77: 11–22. https://doi.org/10.1016/j.dsr.2013.03.002
- Rodríguez Mendoza RP (2006) Otoliths and their applications in fishery science. Croatian Journal of Fisheries: Ribarstvo 64(3): 89–102.
- Ross JR, Crosby JD, Kosa JT (2005) Accuracy and Precision of Age Estimation of Crappies. North American Journal of Fisheries Management 25(2): 423–428. https://doi.org/10.1577/M04-083.1
- Russ JC (1990) Computer-assisted microscopy: The measurement and analysis of images. Plenum Press, New York, 453 pp.
- Sabatés A (2004) Diel vertical distribution of fish larvae during the winter-mixing period in the northwestern Mediterranean. ICES Journal of Marine Science 61(8): 1243–1252. https://doi.org/10.1016/j.icesjms.2004.07.022
- Sardo G, Geraci ML, Scannella D, Falsone F, Vitale S (2020) New records of two uncommon species, Calappa tuerkayana Pastore, 1995 (Decapoda, Calappidae) and Parasquilla ferrussaci (Roux, 1828) (Stomatopoda, Parasquillidae), from the Strait of Sicily (central Mediterranean Sea). Arxius de Miscel·lània Zoològica 18: 113–121. https://doi.org/10.32800/amz.2020.18.0113
- Schaefer S, Johnson RK, Badcock J (1986) Photichthyidae. Pp. 243–247. In: Smith MM, Heemstra PC (Eds) Smiths’ sea fishes. Springer-Verlag, Berlin, Germany.
- Secor DH, Dean JM, Laban EH (1992) Otolith removal and preparation for microstructural examination. Stevenson DK, Campana SE (Eds) Otolith microstructure examination and analysis. Canada Communication Group, Ottawa, 19–57. https://doi.org/10.2307/1446235
- Smale MJ, Watson G, Hecht T (1995) Otolith atlas of southern African marine fishes. Ichthyological Monographs of the J.L.B. Smith Institute of Ichthyology, Vol. 1, 253 pp. https://doi.org/10.5962/bhl.title.141860
- Somarakis S, Isari S, Machias A (2011) Larval fish assemblages in coastal waters of central Greece: Reflections of topographic and oceanographic heterogeneity. Scientia Marina 75(3): 605–618. https://doi.org/10.3989/scimar.2011.75n3605
- Torres GJ, Lombarte A, Morales-Nin B (2000) Sagittal otolith size and shape variability to identify geographical intraspecific differences in three species of genus Merluccius. Journal of the Marine Biological Association of the United Kingdom 80(2): 333–342. https://doi.org/10.1017/S0025315499001915
- Tuset VM, Lozano IJ, González JA, Pertusa JF, García‐Díaz MM (2003) Shape indices to identify regional differences in otolith morphology of comber, Serranus cabrilla (L., 1758). Journal of Applied Ichthyology 19(2): 88–93. https://doi.org/10.1046/j.1439-0426.2003.00344.x
- Tuset VM, Lombarte A, Assis CA (2008) Otolith atlas for the western Mediterranean, north and central eastern Atlantic. Scientia Marina 72(S1): 7–198. https://doi.org/10.3989/scimar.2008.72s17
- Vignon M, Morat F (2010) Environmental and genetic determinant of otolith shape revealed by a non-indigenous tropical fish. Marine Ecology Progress Series 411: 231–241. https://doi.org/10.3354/meps08651
- Watanabe H, Moku M, Kawaguchi K, Ishimaru K, Ohno A (1999) Diel vertical migration of myctophid fishes (Family Myctophidae) in the transitional waters of the western North Pacific. Fisheries Oceanography 8(2): 115–127. https://doi.org/10.1046/j.1365-2419.1999.00103.x
- Weitzman SH (1974) Osteology and evolutionary relationships of the Sternoptychidae with a new classification of stomiatoid families. Bulletin of the American Museum of Natural History 153(3): 327–478.
- Yang J, Huang Z, Chen S, Li Q (1996) [The deep-water pelagic fishes in the area form Nansha Islands to the northeast part of South China Sea. ] Science Publication Company, Beijing, China, 190 pp. [In Chinese]
- Zarrad R, Alemany F, Rodriguez JM, Jarboui O, Lopez-Jurado JL, Balbin R (2013) Influence of summer conditions on the larval fish assemblage in the eastern coast of Tunisia (Ionian Sea, Southern Mediterranean). Journal of Sea Research 76: 114–125. https://doi.org/10.1016/j.seares.2012.08.001